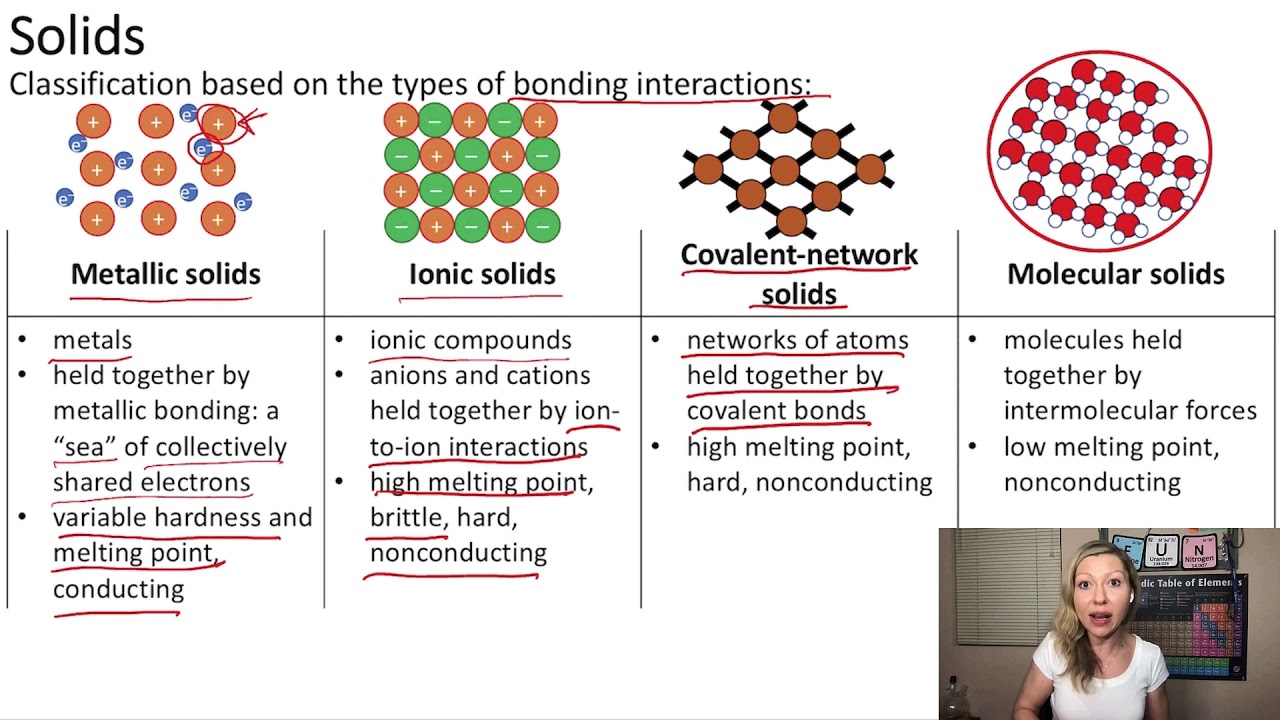
Covalent network solids are fascinating materials that exhibit unique properties due to the strong covalent bonds that hold their atoms together. These solids form a continuous network of atoms, creating a rigid structure that contributes to their distinctive characteristics. Understanding the properties of covalent network solids is essential for various scientific disciplines, including chemistry, materials science, and engineering. Their applications range from industrial uses to everyday products, making them a significant area of study.
The properties of covalent network solids arise from their atomic structure, which is defined by a network of bonds between atoms. Unlike ionic or metallic solids, where electrons are transferred or shared in a more localized manner, covalent network solids maintain a three-dimensional arrangement of atoms bonded by shared electrons. This unique structure imparts various physical and chemical properties, such as hardness, high melting points, and electrical insulating behavior. The study of these properties helps us understand not only the materials themselves but also the underlying principles of chemical bonding and molecular interactions.
In addition to their intriguing structural characteristics, covalent network solids play crucial roles in various applications, including semiconductors, optical devices, and nanotechnology. As researchers continue to explore these materials, they uncover new possibilities for innovation and technological advancement. This article will delve deeper into the properties of covalent network solids, addressing key questions that will enhance our understanding of their significance in both scientific research and practical applications.
What Are the Key Properties of Covalent Network Solids?
Covalent network solids possess several key properties that distinguish them from other types of solids. Here are some of the most notable characteristics:
- Hardness: Covalent network solids are typically very hard due to the strong covalent bonds that create their structure. Diamond, for example, is one of the hardest known materials.
- High Melting and Boiling Points: The melting and boiling points of covalent network solids are generally high because breaking the strong covalent bonds requires a significant amount of energy.
- Poor Electrical Conductivity: Most covalent network solids are insulators, as they lack free electrons that can move and conduct electricity.
- Insolubility: Many covalent network solids are insoluble in common solvents due to the strong interatomic forces that hold their structures together.
How Do Covalent Network Solids Compare to Other Types of Solids?
To appreciate the unique properties of covalent network solids, it's essential to compare them with other types of solids, such as ionic and metallic solids. Here’s a breakdown of the differences:
Covalent Network Solids vs. Ionic Solids
Ionic solids consist of positively and negatively charged ions held together by ionic bonds. Unlike covalent network solids, ionic solids tend to be brittle and have lower melting points. When dissolved in water, ionic solids can conduct electricity due to the movement of ions, while covalent network solids cannot.
Covalent Network Solids vs. Metallic Solids
Metallic solids are characterized by a "sea of electrons" that allows them to conduct electricity and heat efficiently. In contrast, covalent network solids do not have this free electron movement, which contributes to their insulating properties and rigidity. While metallic solids are malleable and ductile, covalent network solids are typically brittle.
What Are Some Common Examples of Covalent Network Solids?
Several well-known materials fall under the category of covalent network solids, each showcasing the distinct properties associated with this type of bonding. Some prominent examples include:
- Diamond: Composed entirely of carbon atoms arranged in a tetrahedral lattice, diamond is renowned for its unparalleled hardness and brilliance.
- Silicon Carbide (SiC): This compound exhibits exceptional thermal conductivity and is used in high-performance applications such as abrasives and semiconductors.
- Graphite: Although graphite is composed of carbon atoms, its layered structure allows for electrical conductivity along the planes, making it an interesting example of covalent bonding.
- Quartz (SiO2): A common mineral, quartz forms a crystalline structure that is both hard and resistant to chemical weathering.
Why Are Covalent Network Solids Important in Technology?
The unique properties of covalent network solids make them integral to various technological applications. Their high hardness and thermal stability make them suitable for cutting tools and abrasives. Additionally, materials like silicon carbide are essential in the electronics industry for their semiconductor properties. Understanding these materials allows scientists and engineers to innovate and improve technologies across multiple fields.
How Do Covalent Network Solids Influence Material Science?
In material science, the study of covalent network solids contributes to the development of new materials with tailored properties. By manipulating the atomic structure and bonding arrangements, researchers can create materials that exhibit specific characteristics suitable for various applications. This ongoing research has the potential to lead to breakthroughs in fields such as nanotechnology, renewable energy, and advanced manufacturing.
What Challenges Do Scientists Face When Studying Covalent Network Solids?
Despite the fascinating properties of covalent network solids, scientists encounter several challenges in their study and application. Some of these challenges include:
- Characterization: Accurately characterizing the complex structures of covalent network solids can be difficult, requiring advanced techniques and equipment.
- Scalability: Producing large quantities of high-quality covalent network materials can be challenging, especially for applications like semiconductors.
- Understanding Behavior: Predicting the behavior of covalent network solids under different conditions still poses a scientific challenge, necessitating ongoing research.
What Future Developments Can We Expect in Covalent Network Solid Research?
As research in covalent network solids continues to evolve, we can anticipate exciting developments. Innovations may include:
- New Materials: The creation of novel covalent network solids with tailored properties for specific applications.
- Improved Production Techniques: Advancements in fabrication methods to enhance the scalability and quality of covalent network materials.
- Enhanced Understanding: Greater insights into the behavior and interactions of covalent network solids, leading to improved applications in technology.
In conclusion, the properties of covalent network solids are pivotal in both understanding the fundamental aspects of materials science and driving technological advancements. As researchers continue to explore these fascinating materials, we can expect new discoveries that will impact various fields and improve our daily lives.
Article Recommendations
- G3 Case
- Vintage Grandfather Wall Clock
- Sherell Ford
- Night Of The Living Deb Script
- Evergreen Bushes And Shrubs
- Proofreading Payment
- How To Turn Off Volte
- Goldman Sachs Pwm Associate Salary
- Old Dollar Shave Club Handle
- Gta Iv Script Hook